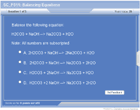
Balancing Equations
| Topic Review on "Title": |
Chemical reactions are balanced with coefficients until the numbers of each atom are equal on the left and the right.
The Law of Conservation of Mass
The Law of Conservation of Mass states that the mass of the reactants equal the mass of the products. Atoms cannot be created nor destroyed in a chemical change—therefore, the number of each type of atom on each side of the reaction must be equal. Coefficients are used to balance chemical reactions.
Choosing which atom to start with
Start with elements that appear only one time on each side and elements that are in the most complex compounds. End with elements that appear more than once on a side or elements that appear uncombined on one side or the other.
Inspection method of balancing
The Inspection Method is used to balance the simplest reactions. It includes:
- Make a list of the elements in the reaction
- Count the number of each type of atom on each side of the reaction
- Add coefficients to balance the number of atoms
- Determine the total charge of each side of the reaction and use coefficients to balance charge.
- When all elements and charge are balanced, place a “1” in any empty coefficient location to indicate that you’re done.
|
| Rapid Study Kit for "Title": |
| Flash Movie |
Flash Game |
Flash Card |
| Core Concept Tutorial |
Problem Solving Drill |
Review Cheat Sheet |
 |
 |
 |
|
| "Title" Tutorial Summary : |
The Law of Conservation of Matter says that matter cannot be created nor destroyed. That means that the atoms that are on the reactant side also must appear on the products side. Therefore, reactions need to be balanced. This tutorial shows methods that can be used to balance simple reactions.
|
| Tutorial Features: |
Specific Tutorial Features:
- Instructions on how to choose the atom to begin with is given
- Multiple methods of balancing equations are worked out in animation.
Series Features:
- Concept map showing inter-connections of new concepts in this tutorial and those previously introduced.
- Definition slides introduce terms as they are needed.
- Visual representation of concepts
- Animated examples—worked out step by step
- A concise summary is given at the conclusion of the tutorial.
|
| "Title" Topic List: |
- Using The Law of Conservation of Mass to balance the equations
- Choosing which atom to begin balancing with
- Inspection method of balancing
|
See all 24 lessons in college chemistry, including concept tutorials, problem drills and cheat sheets:
Teach Yourself SAT Chemistry Visually in 24 Hours |



